
Introduction of Hydrogen Chloride
hydrogen chloride (HCl), a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. A solution of the gas in water is called hydrochloric acid.
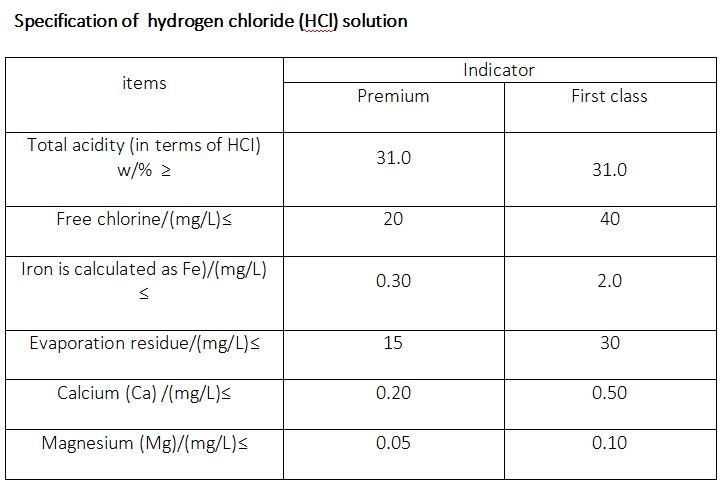
Production of Hydrogen Chloride
Hydrogen chloride may be formed by the direct combination of chlorine (Cl2) gas and hydrogen (H2) gas; the reaction is rapid at temperatures above 250 °C (482 °F). The reaction, represented by the equation H2 + Cl2 → 2HCl, is accompanied by evolution of heat and appears to be accelerated by moisture. Hydrochloric acid is prepared by dissolving gaseous hydrogen chloride in water. Because of the corrosive nature of the acid, ceramic, glass, or sometimes tantalum apparatus is commonly used. Hydrochloric acid is usually marketed as a solution containing 28–35 percent by weight hydrogen chloride, commonly known as concentrated hydrochloric acid.
Physical properties of Hydrogen Chloride
Hydrogen chloride is a colourless gas of strong odour. It condenses at ?85 °C (?121 °F) and freezes at ?114 °C (?173 °F). The gas is very soluble in water: at 20 °C (68 °F) water will dissolve 477 times its own volume of hydrogen chloride. Because of its great solubility, the gas fumes in moist air. A water solution containing 20.24 percent by weight hydrogen chloride boils at 110 °C (230 °F) without change in composition (azeotropic mixture). In aqueous solution the compound is extensively dissociated into a hydronium ion (H3O+) and chloride ion (Cl?); in dilute solutions the dissociation is essentially complete. Thus, hydrochloric acid is a strong acid.
Chemical properties of Hydrogen Chloride
Acid titration.
Hydrogen chloride (HCl) is a monoprotic acid, which means that each molecule can dissociate (ionize) only once to release one H+ ion (a single proton). In aqueous hydrochloric acid, the H+ joins a water molecule to form a hydronium ion, H3O+:
HCl + H2O ? H3O+ + Cl?
Molecular model of hydrogen chloride.
The other ion formed is Cl?, the chloride ion. Hydrochloric acid can therefore be used to prepare salts called chlorides, such as sodium chloride. Hydrochloric acid is a strong acid, since it is fully dissociated in water.
Applications of Hydrogen Chloride
Hydrochloric acid is a strong inorganic acid that is used in many industrial processes. The application often determines the required product quality.It is routinely used in chemical research laboratories and manufacturing plants. Its applications include the large-scale production of certain compounds (such as vinyl chloride for polyvinyl chloride (PVC) plastic), removal of rust and scale from metals, petroleum production, and ore processing. Smaller-scale applications include the production of gelatin and other ingredients in food, and leather processing. An estimated 20 million metric tons of hydrochloric acid are produced annually.
Regeneration of ion exchangers
An important application of high-quality hydrochloric acid is the regeneration of ion exchange resins. Cation exchange is widely used to remove ions such as Na+ and Ca2+ from aqueous solutions, producing demineralized water.
Na+ is replaced by H3O+
Ca2+ is replaced by 2 H3O+
Ion exchangers and demineralized water are used in all chemical industries, drinking water production, and many food industries.
pH Control and neutralization
A very common application of hydrochloric acid is to regulate the basicity (pH) of solutions.
OH? + HCl → H2O + Cl?
In industry demanding purity (food, pharmaceutical, drinking water), high-quality hydrochloric acid is used to control the pH of process water streams. In less-demanding industry, technical-quality hydrochloric acid suffices for neutralizing waste streams and swimming pool treatment.
Pickling of steel
Pickling is an essential step in metal surface treatment, to remove rust or iron oxide scale from iron or steel before subsequent processing, such as extrusion, rolling, galvanizing, and other techniques. Technical-quality HCl at typically 18 percent concentration is the most commonly-used pickling agent for the pickling of carbon steel grades.
Fe2O3 + Fe + 6 HCl → 3 FeCl2 + 3 H2O
The spent acid has long been re-used as ferrous chloride solutions, but high heavy-metal levels in the pickling liquor has decreased this practice.
In recent years, the steel pickling industry has however developed hydrochloric acid regeneration processes, such as the spray roaster or the fluidized bed HCl regeneration process, which allow the recovery of HCl from spent pickling liquor.
Production of inorganic compounds
Numerous products can be produced with hydrochloric acid in normal acid-base reactions, resulting in inorganic compounds. These include water treatment chemicals such as iron(III) chloride and polyaluminium chloride (PAC).
Fe2O3 + 6 HCl → 2 FeCl3 + 3 H2O
Both iron(III) chloride and PAC are used as flocculation and coagulation agents in wastewater treatment, drinking water production, and paperproduction.
Other inorganic compounds produced with hydrochloric acid include road application salt calcium chloride, nickel(II) chloride for electroplating, and zinc chloride for the galvanizing industry and battery production.
Production of organic compounds
The largest hydrochloric acid consumption is in the production of organic compounds such as vinyl chloride for PVC, and MDI and TDI for polyurethane. This is often captive use, consuming locally-produced hydrochloric acid that never actually reaches the open market. Other organiccompounds produced with hydrochloric acid include bisphenol A for polycarbonate, activated carbon, and ascorbic acid, as well as numerous pharmaceutical products.
Other applications
Hydrochloric acid is a fundamental chemical, and as such it is used for a large number of small-scale applications, such as leather processing, household cleaning, and building construction. In addition, a way of stimulating oil production is by injecting hydrochloric acid into the rock formation of an oil well, dissolving a portion of the rock, and creating a large-pore structure. Oil-well acidizing is a common process in the North Sea oil production industry.
Many chemical reactions involving hydrochloric acid are applied in the production of food, food ingredients, and food additives. Typical products include aspartame, fructose, citric acid, lysine, hydrolyzed (vegetable) protein as food enhancer, and in gelatin production. Food-grade (extra-pure) hydrochloric acid can be applied when needed for the final product.

湘ICP備16009356號-1 恒光股份 TEL:400-800-3766